This science experiment is so mesmerizing, you won’t be able to stop doing it once you start! The experiment only requires five, household items, and it is tons of fun!
For this experiment, you will need:

- small plate or bowl
- milk (preferably 2% or whole, but 1% works as well)
- liquid food coloring
- dish soap
- Q-tip
Steps of the experiment:
- Pour enough milk into your bowl or plate to cover its bottom

- Drop different colors of food coloring beside each other on the surface of the milk

- Coat the end of the Q-tip in dish soap

- Press the soapy end of the Q-tip in the intersection of the drops of food coloring for 10-15 seconds
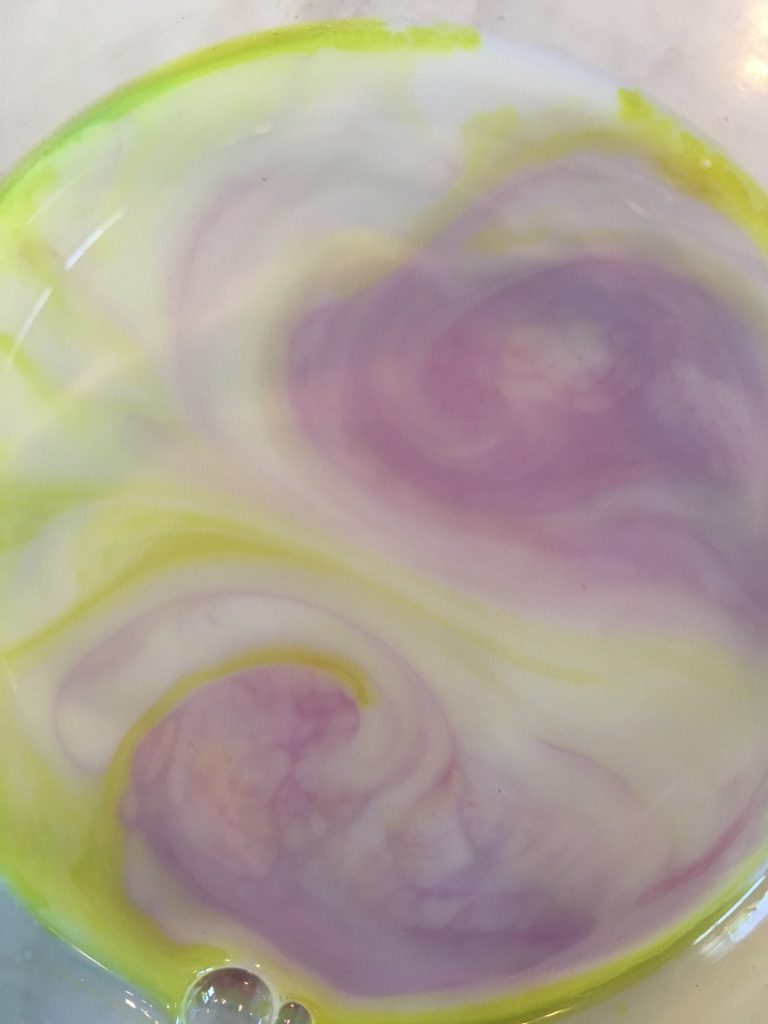
- Watch science happen!
What makes the color swirl?
Milk is made mostly of water, but tiny drops of fat and protein are suspended in milk. Milk fat and protein are hydrophobic molecules, meaning they do not dissolve in water. Dish soap, because of its bipolar characteristics (nonpolar on one end and polar on the other), weakens the chemical bonds that hold the proteins and fats in milk. The soap’s hydrophilic, or water-loving, end dissolves in water, and its hydrophobic, or water-fearing, end attaches to a fat globule in the milk. The molecules of fat bend and twist in different directions as the soap molecules race around to join up with the fat molecules. During all of this, the food coloring molecules are bumped everywhere, creating the color swirls we can observe. As the soap becomes evenly mixed with the milk, the action slows down and eventually stops. This is why milk with a higher fat content produces a better explosion of color—there’s more fat to combine with the soap molecules.

Leila
Can’t wait to do this with the kids! They will really enjoy.
Julia
Thanks! It’s a really fun experiment!
DeKiya
You have a gorgeous cover photo!
Julia
Thank you!